
Blacksmithing
and Cutlery
by Gérard HEUTTE







|
|
 General points about heat treating
General points about heat treating

|
Heat treating is essential to make a good blade! This page presents some information
to be known before seeing in detail the main heat treatments.
 Bases
Bases
The term "Heat treatment" indicates operations
of heating and cooling, controlled in time, in order to give to steel
properties adapted to the future use.
The heat treating acts on the macroscopic structure and
the mechanical state of steel, without modifying its composition.
The traditional and artisanal metallurgy calls mainly upon
continuous cooling. The modern metallurgy techniques use more and more
isothermal treatments.
 Temperature and color
Temperature and color
For heating, the observation of steel color
remains a good indicator. To make this method
reliable, it is necessary to observe steel in the half-light.
It should however be noted that the interpretation of the
colors may vary from one individual to another!
The table below is thus given as an indication...
Since antiquity, generations of blacksmiths have
made remarkable objects with these bench marks!
| Temperature |
Color |
Example |
| 550°C / 1022°F |
Brown |
|
| 660°C / 1220°F |
Dark red |
|
| 780°C / 1436°F |
Red |
|
| 860°C / 1580°F |
Red Orange |
|
| 930°C / 1706°F |
Orange |
|
| 990°C / 1814°F |
Orange Clear |
|
| 1050°C / 1922°F |
Yellow |
|
| 1200°C / 2192°F |
White |
|
 Curie temperature and loss of magnetism
Curie temperature and loss of magnetism
The Curie temperature is the temperature above which steel becomes paramagnetic (i.e. the part
will not be attracted by a magnet). The matter is in disorder state in terms of magnetic spin
(not atomic structure). This temperature is 770°C
for steel. This loss of magnetism is reversible when the temperature goes back below the Curie
temperature.
This physical property is a good point of reference. For non-allied steel with a carbon content
higher than 0.65%, the Curie temperature can be used to determine hardening temperature (usually
just above Curie temperature). Below
0.65% or for allied steels, the hardening temperature is higher. For allied steel or non allied
steels with a carbon content less than 0.65%, the Curie temperature will be a good basis,
but the part must be heated again to reach a higher temperature. Here, the experience will
be useful.
For practical aspect, a powerful magnet is chosen. Small attractions should also be detected.
Personally, I suspend my magnet at the end of a string around the forge. Approaching the part,
I can check the loss of magnetism quite fine, observing the deviations of the magnet. I have
thus increased sensitivity compared to a magnet held in the hand while avoiding thermal
radiation! For intense use, think cool the magnet occasionally with a little cold water.
Put again the part into the to fire for a few moments before hardening.
 Thermal cycles
Thermal cycles
The heat treatments are made via thermal cycles.
A thermal cycle is a combination of the temperature and
time factors. In other words, it is a temperature curve according to
time.
In detail of the heat treatments, I provided typical cycles.
In order to facilitate interpretation of them, I use the
following notations:
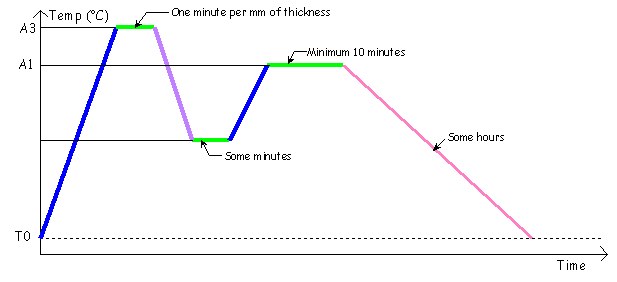
> Blue line: Moderated heating, typically in a forge fire or a gas forge.
> Green line: Stage, keep at constant
temperature.
> Pink line: Very slow cooling, typically in
vermiculite, sand, or ash.
> Purple line: Moderated cooling, typically in the
air.
> Red line: Fast cooling, typically in water, oil or
goop.
Here is a thermal example of cycle (absolutely eccentric!) :
 Heat treatments
Heat treatments
The heat treatments used in cutlery are successively:
> Annealing which
is used to remove the internal stresses.
> Normalization
to refine steel grain.
> Hardening which
hardens steel, but makes it fragile.
> Tempering which
removes much brittleness due to hardening at the detriment of a bit of hardness.
| | |


